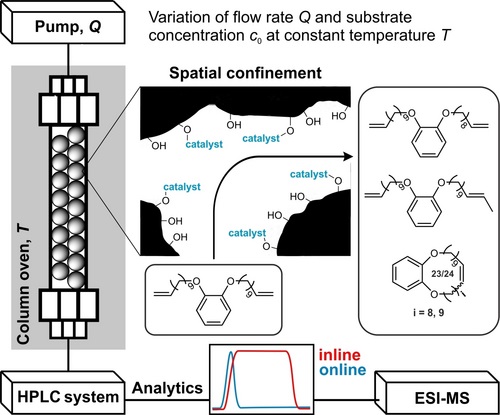
B02 – Online-Kinetic studies of flow-reactions under confinement
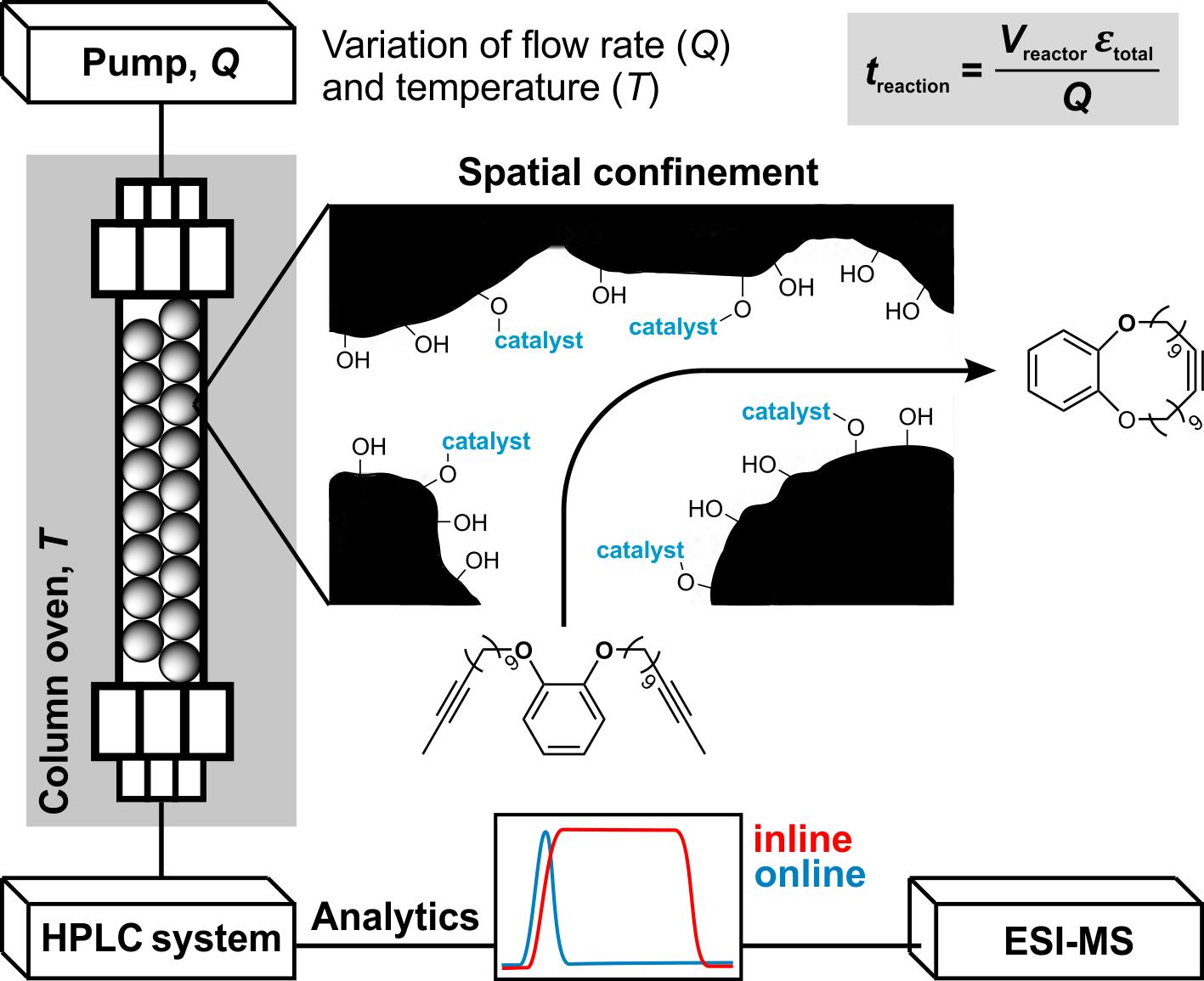
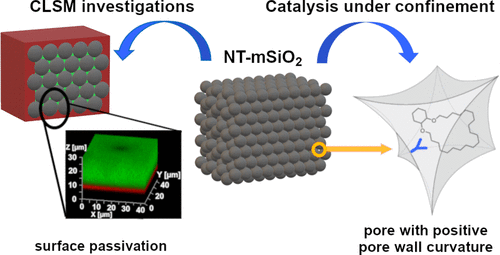
Continuous-flow microreactors and multidimensional on-line analytics for catalytic reaction kinetics studies under spatial confinement
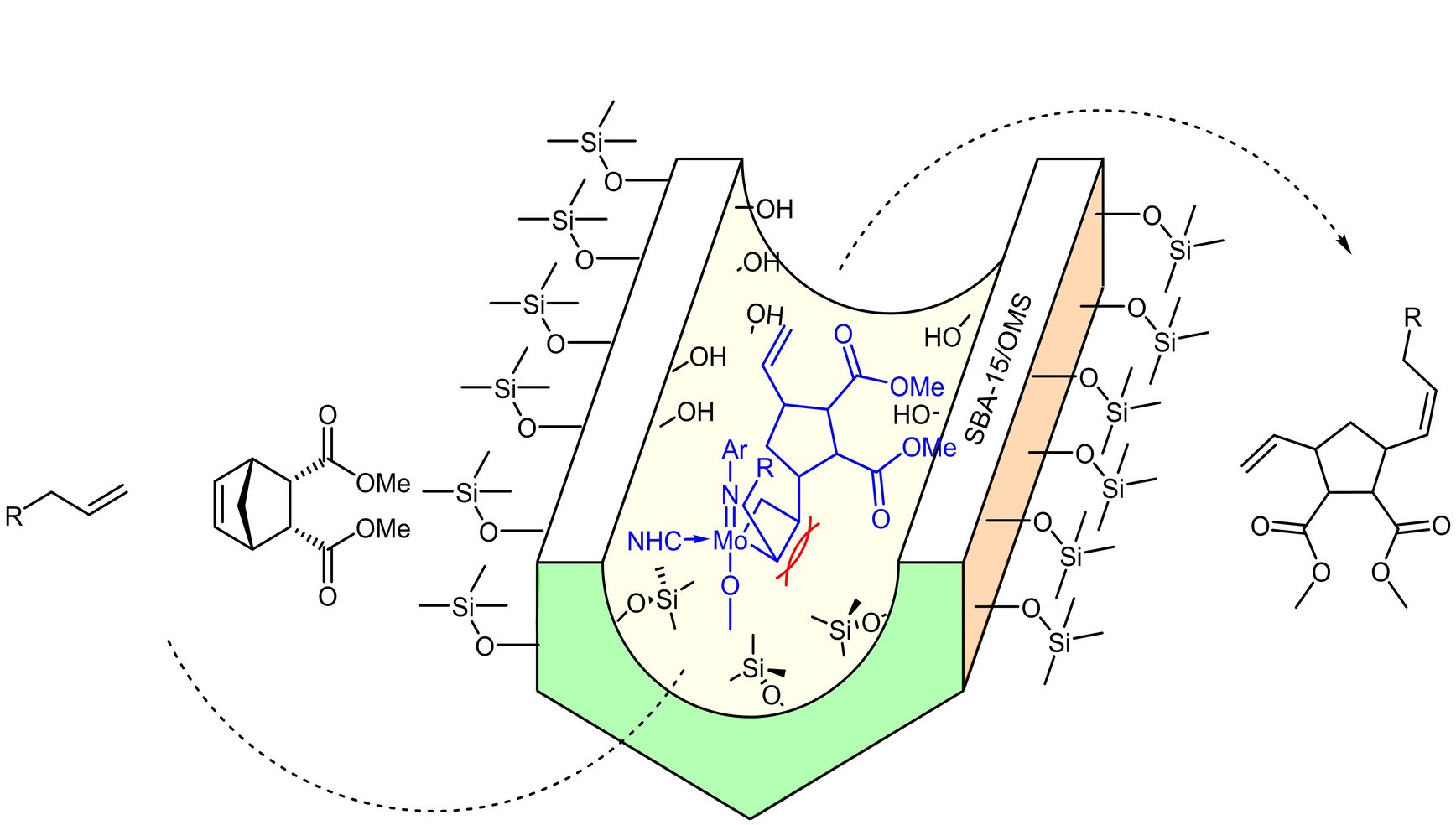
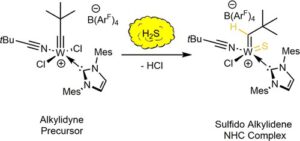
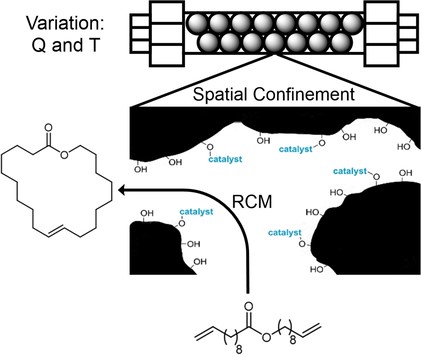
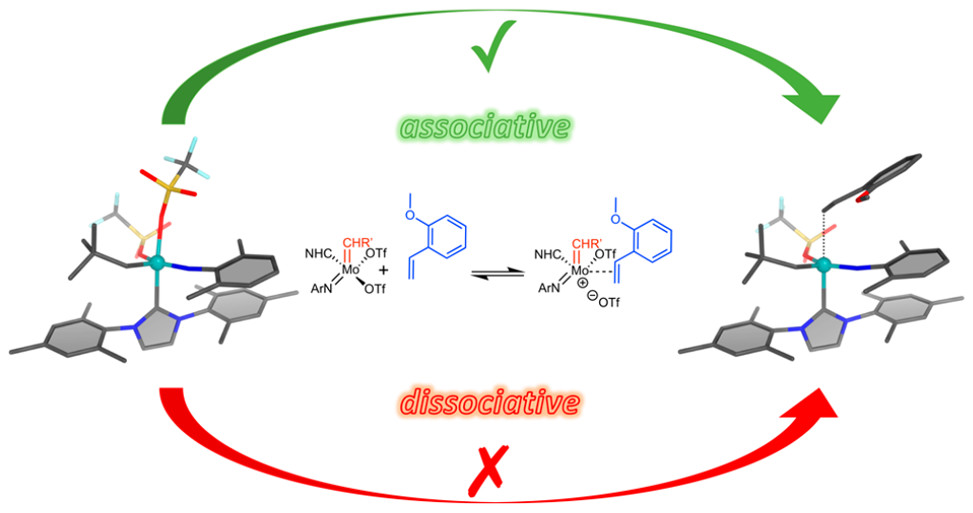
We will study Mo-alkylidyne N-heterocyclic carbene (NHC) catalyzed, alkyne metathesis-based macrocyclization and (Z) selective Rh NHC-catalyzed hydrosilylation and hydroamination reactions of alkynes, all under confinement. The overarching idea in the use of these entirely different catalytic reactions is to develop a unifying concept for confinement effects in catalytic reactions with both large and small substrates. Reactions will be run in continuous-flow microreactors. An instrumental platform with on-line separation and detection will be established to quantify conversion and selectivity under the respective reaction conditions and to determine reaction kinetics rapidly and reproducibly.
Research focus in the 1st funding period (2018-2022):
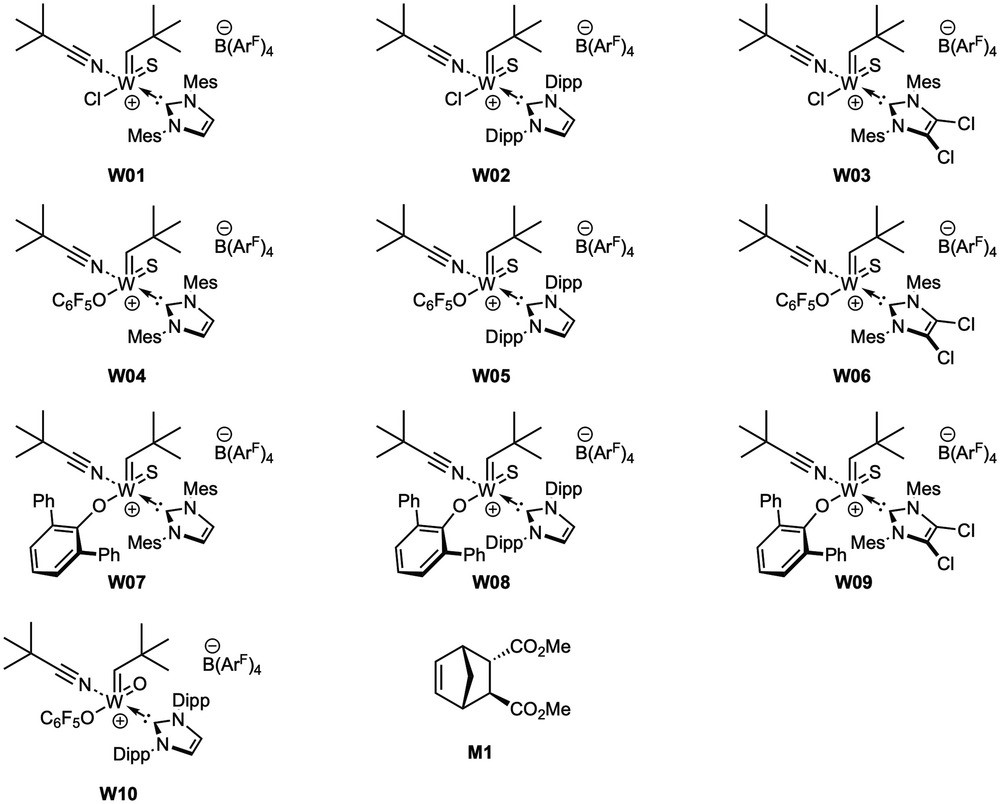
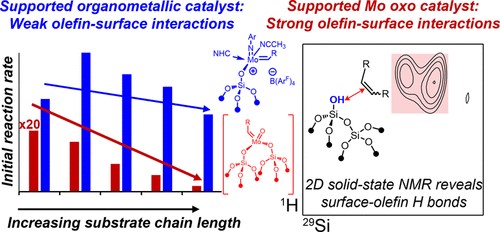
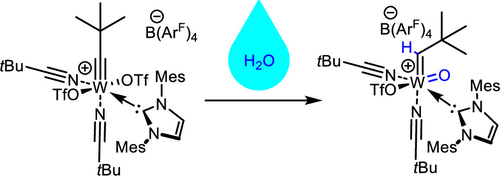
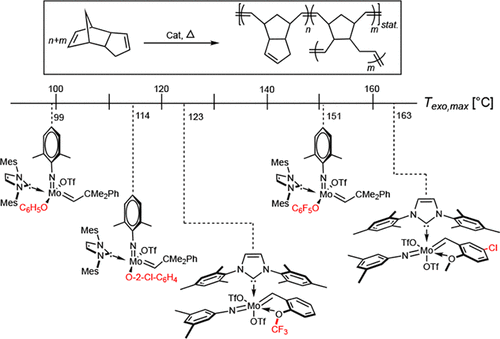
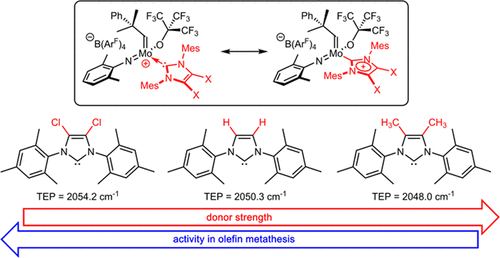
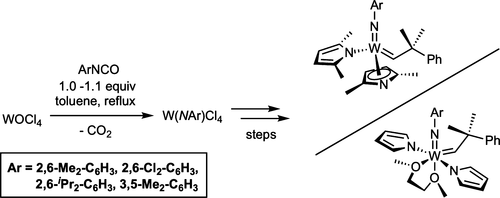
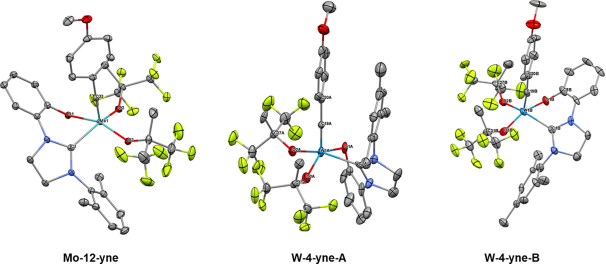
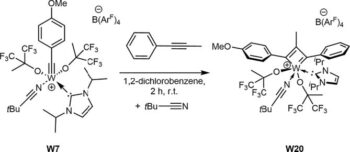
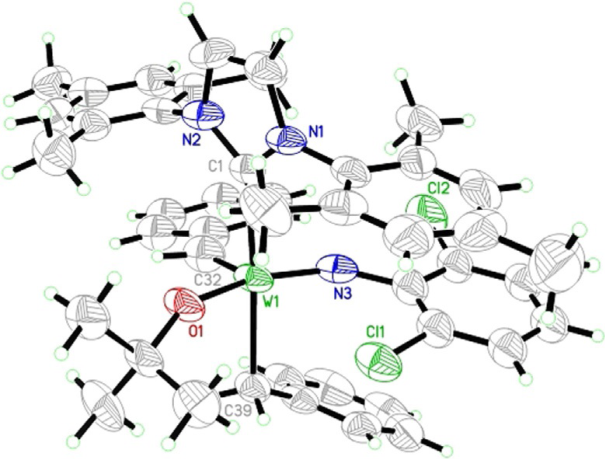
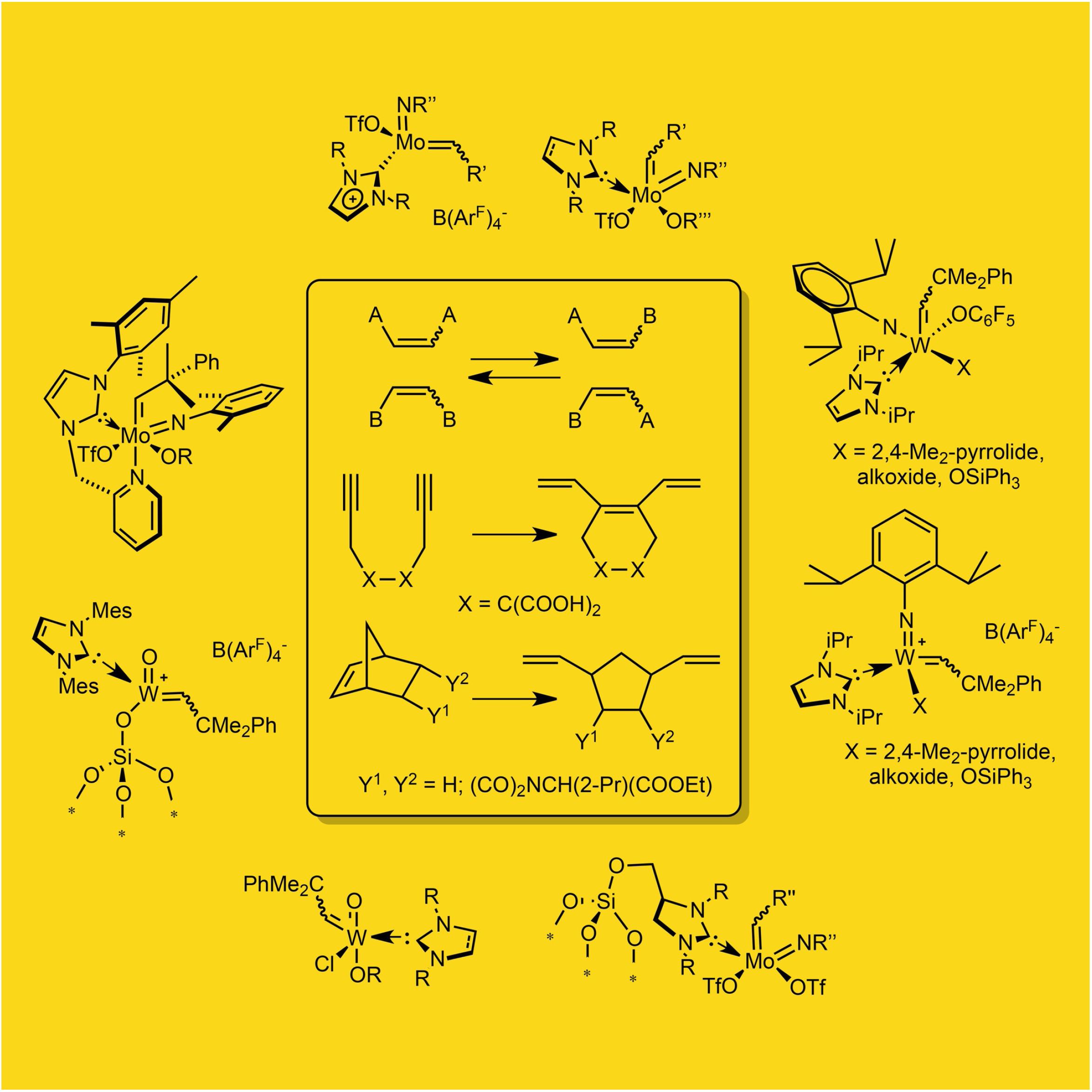
Immobilized Molybdenum Imido, Tungsten Imido- and Tungsten Oxo Alkylidene N-Heterocyclic Carbene Complexes for Olefin Metathesis
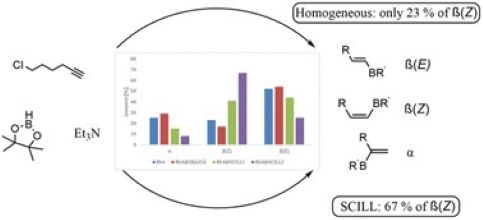
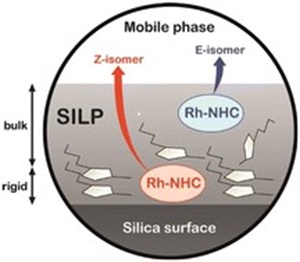
Confinement-induced Z-selectivity in the rhodium N-heterocyclic carbene-catalyzed hydroboration of terminal alkynes
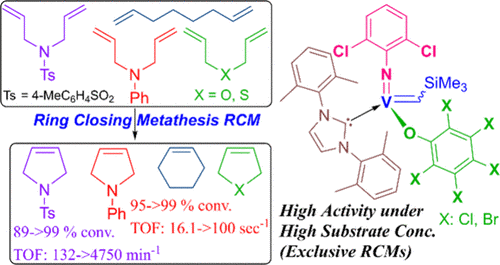
Efficient Ring Closing Metathesis by NHC-Supported(Arylimido)vanadium(V)-Alkylidene Catalysts ContainingPerhalophenoxide Ligands: Exclusive RCM under High SubstrateConcentration
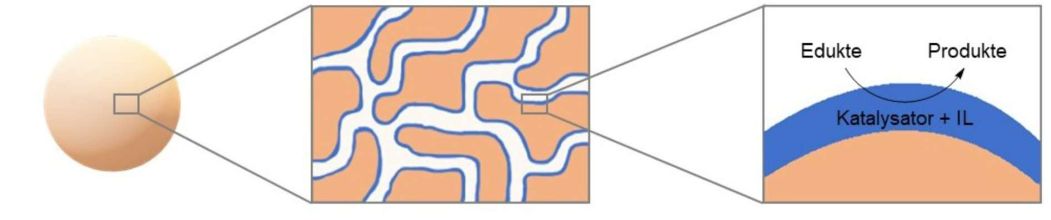
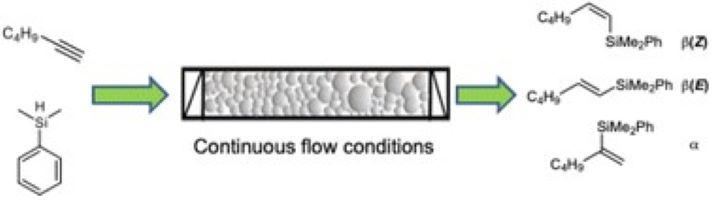
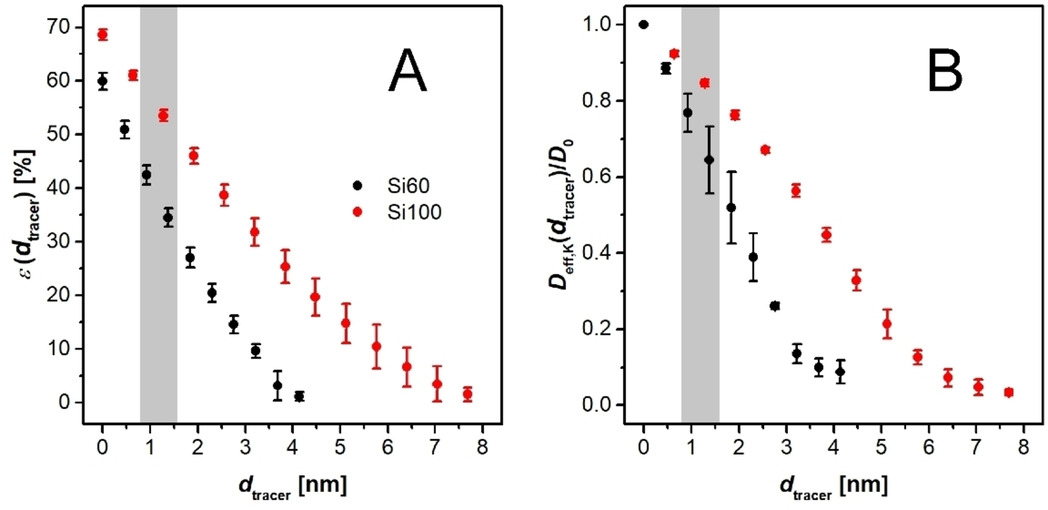
Influence of the supported ionic-liquid layer thickness on Z-selectivity in 1-alkyne hydrosilylation under continuous flow
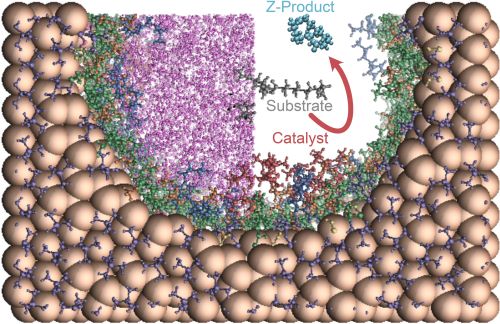
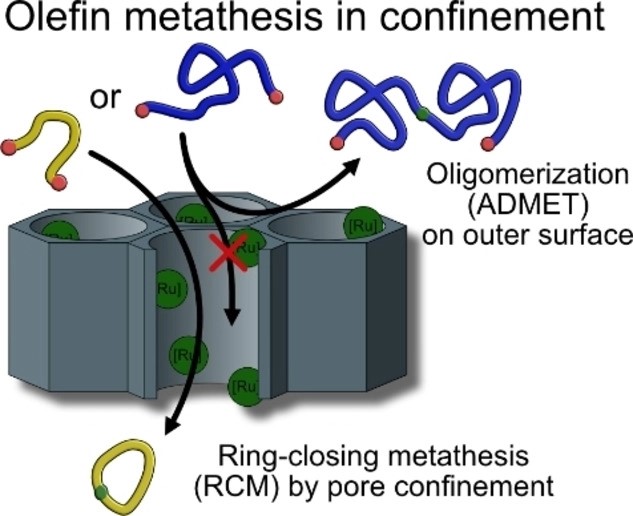
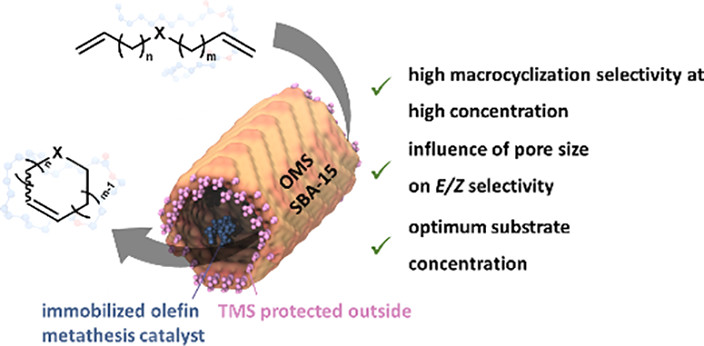
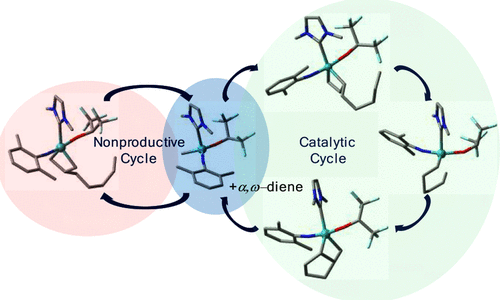
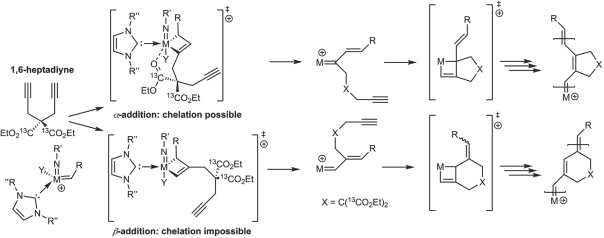
Influence of Ionic Liquid Film Thickness and Flow Rate on Macrocyclization Efficiency and Selectivity in Supported Ionic Liquid-Liquid Phase Catalysis
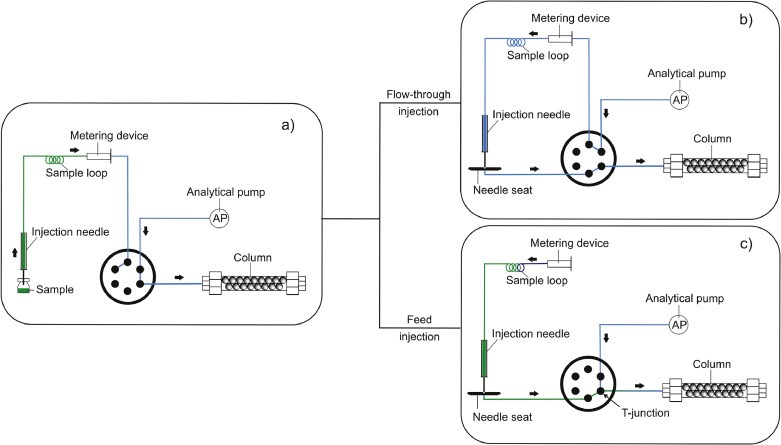
Feed injection in liquid chromatography: Reducing the effect of large-volume injections from purely organic diluents in reversed-phase liquid chromatography
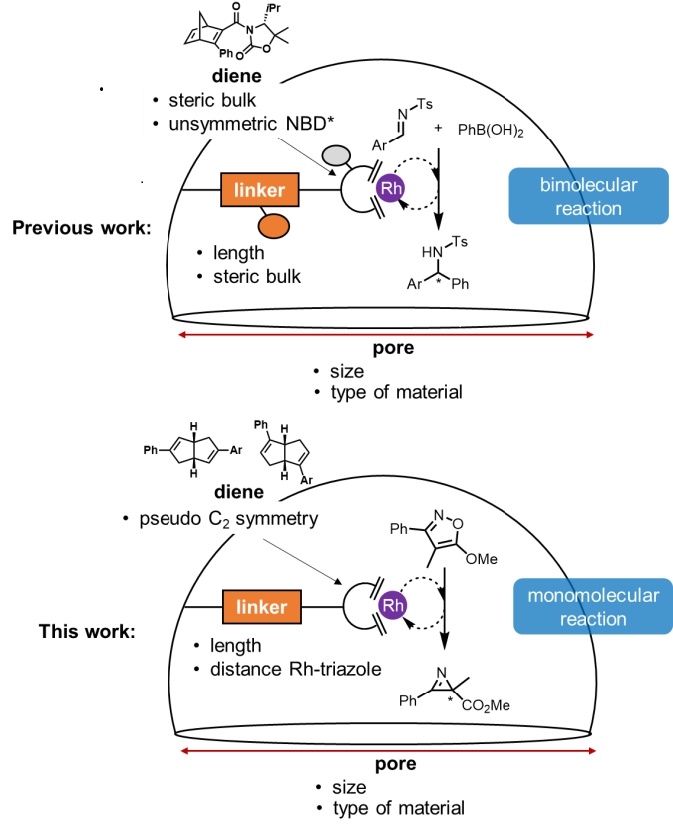
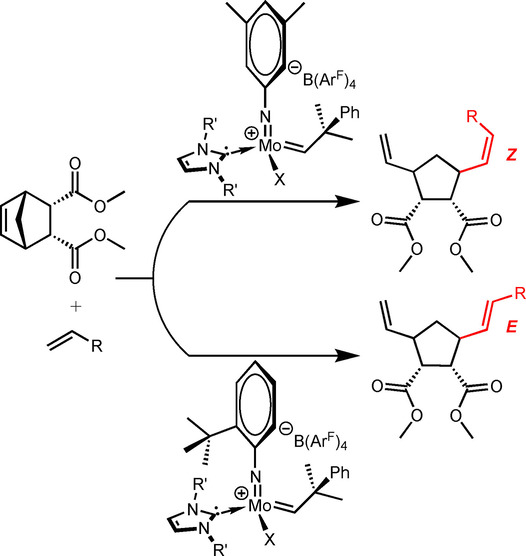
Asymmetric Rh Diene Catalysis under Confinement: Isoxazole Ring-Contraction in Mesoporous Solids
M. Marshall, Z. Dilruba, A.-K. Beurer, K. Bieck, S. Emmerling, F. Markus, Ch. Vogler, F. Ziegler, M. Fuhrer, S. S. Y. Liu, S. R.Kousik, W. Frey, Y. Traa, J. R. Bruckner, B. Plietker, M. R. Buchmeiser, S. Ludwigs, S. Naumann, P. Atanasova, B. V. Lotsch, A. Zens and S. Laschat
Eur. J. Org. Chem. 2024, 27, e202400283.
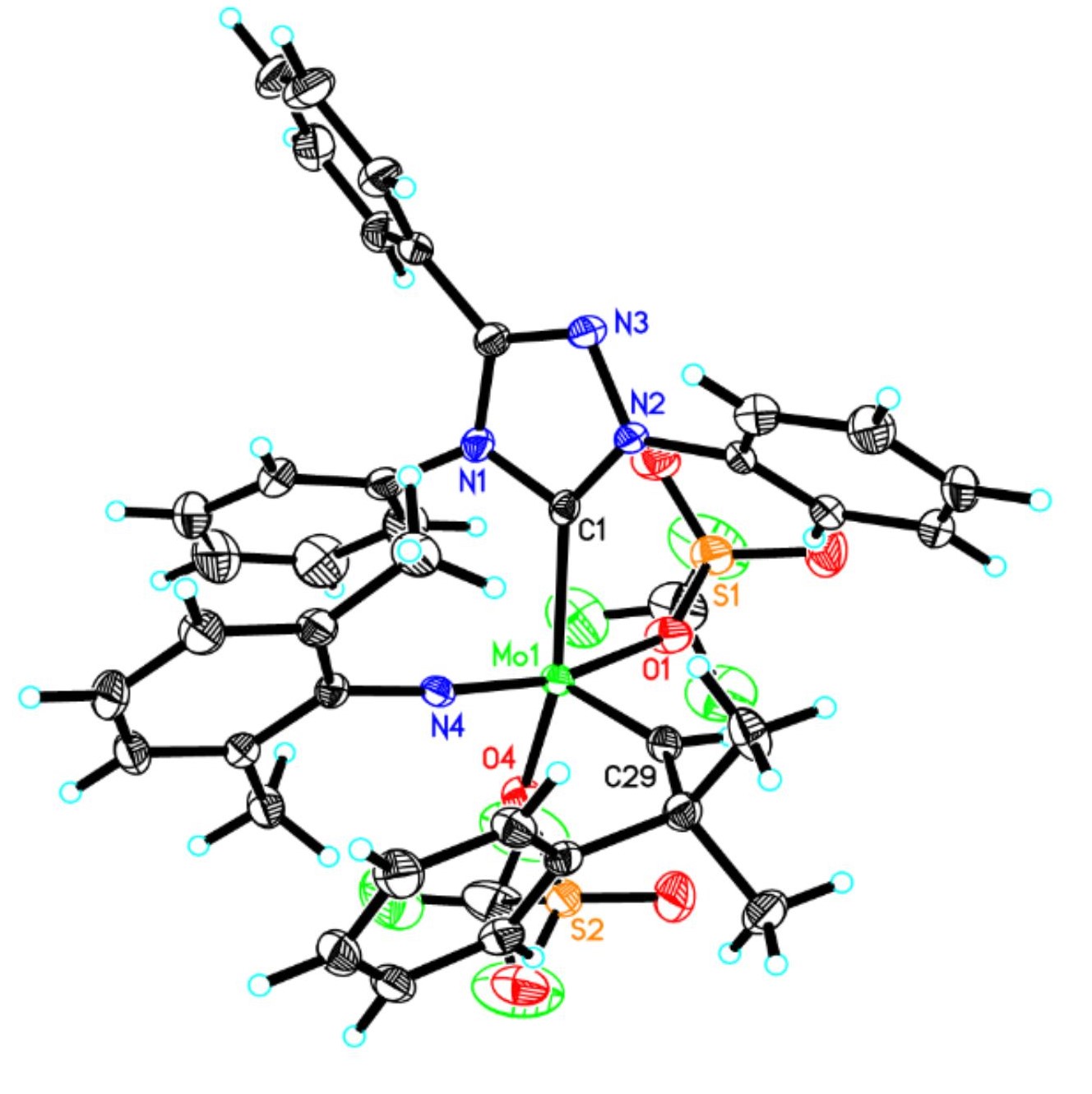
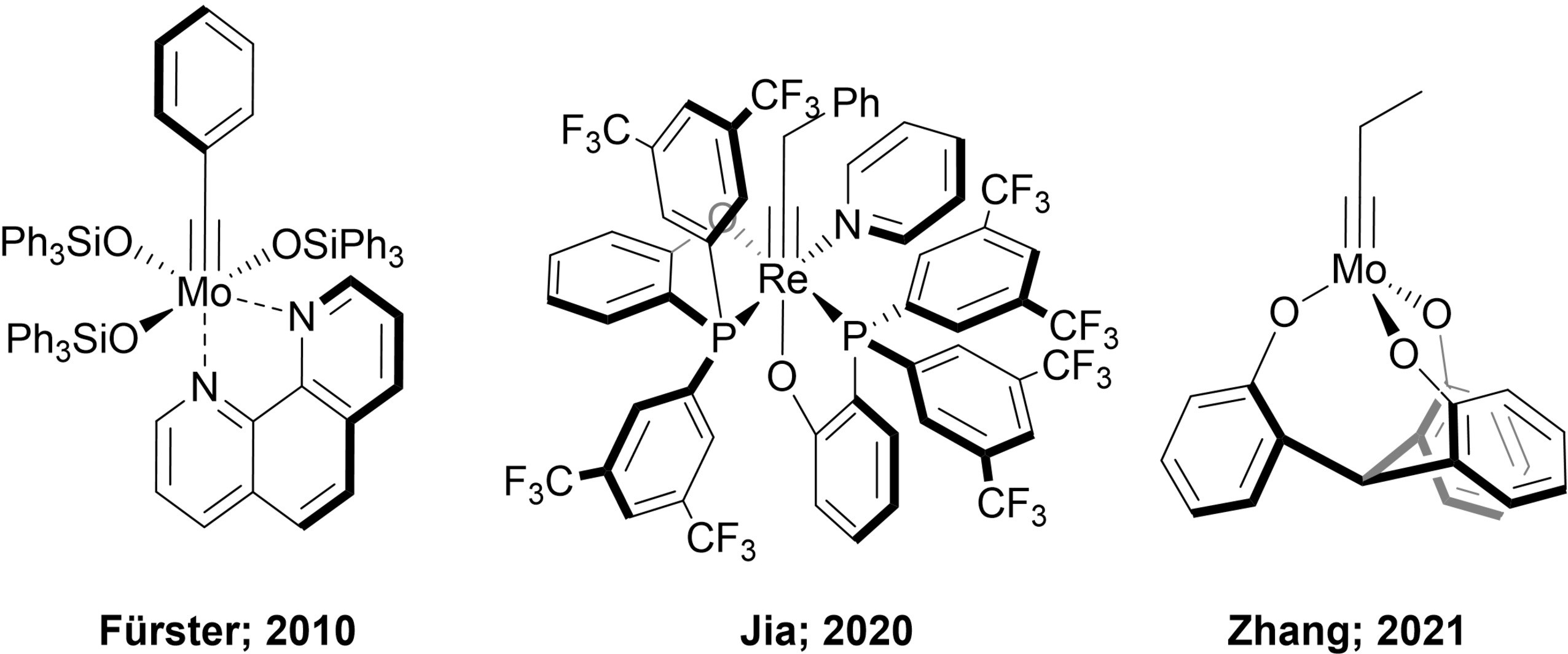
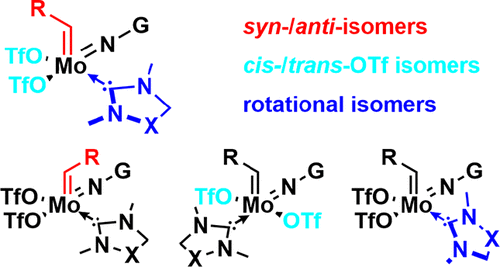
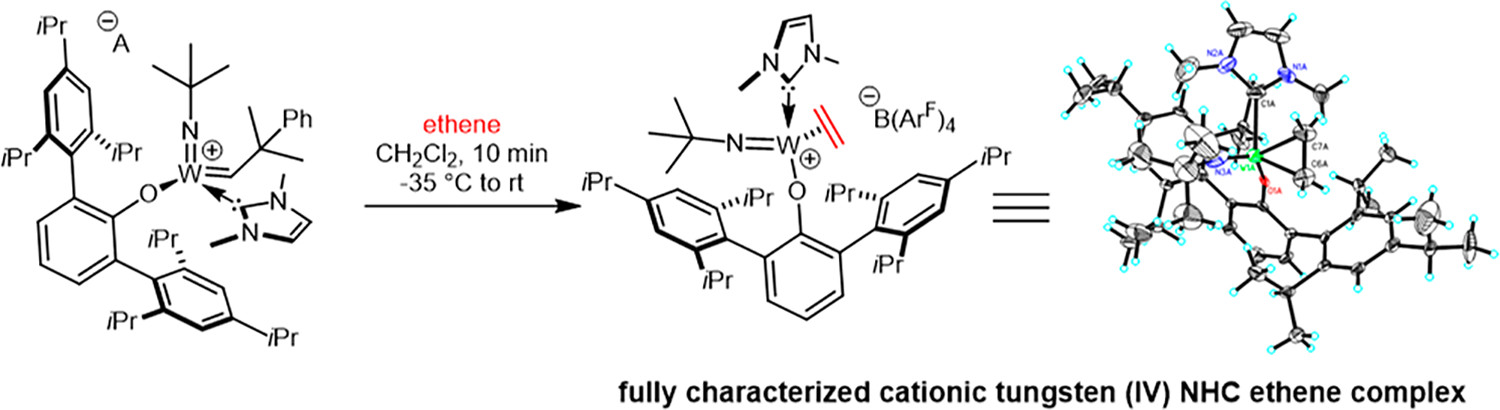
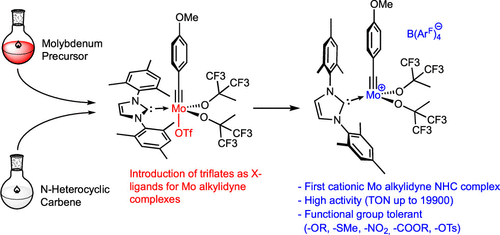
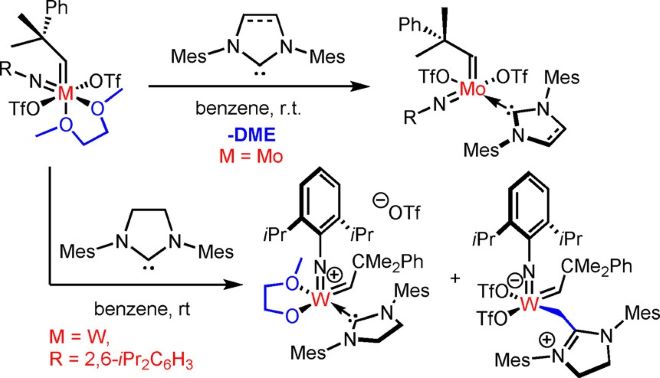
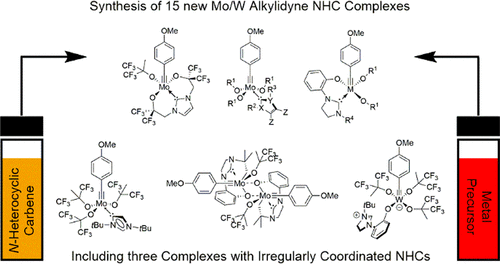
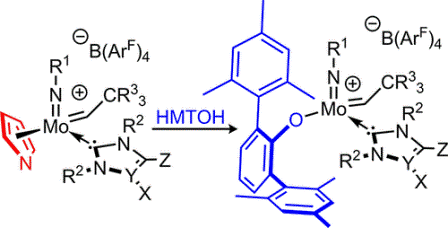
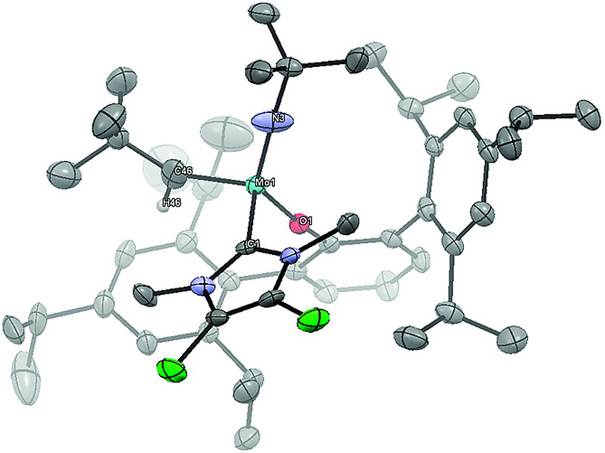
Synthetic and Structural Peculiarities of Neutral and Cationic Molybdenum Imido and Tungsten Oxo Alkylidene Complexes Bearing Weakly Coordinating N-Heterocyclic Carbenes
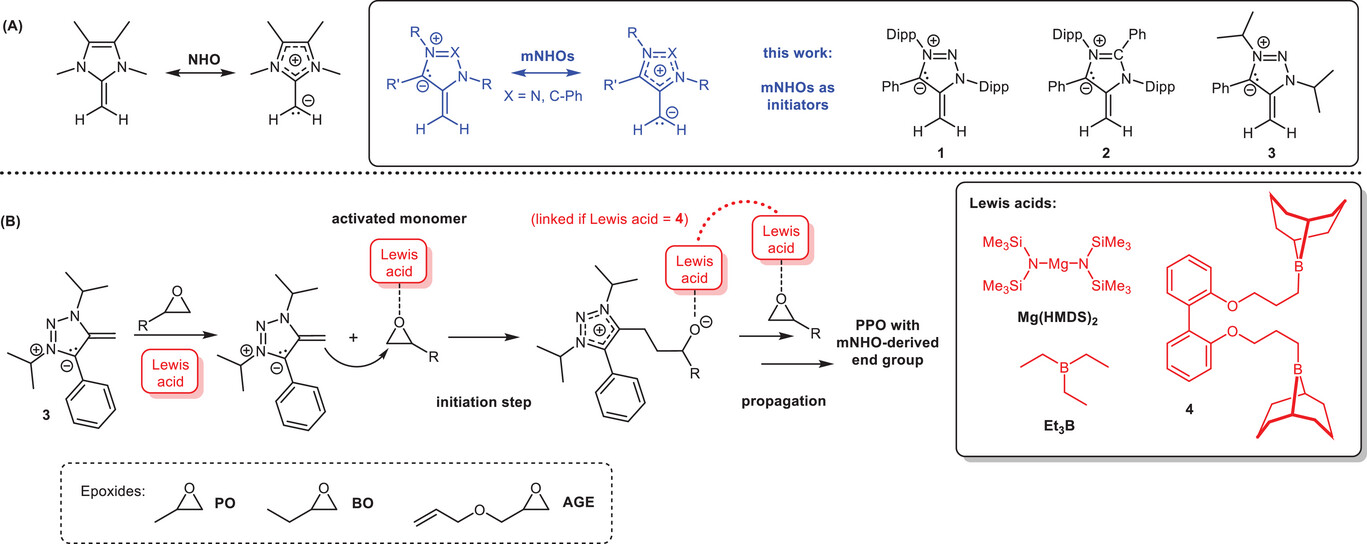
Mesoionic N-Heterocyclic Olefins as Initiators for the Lewis Pair Polymerization of Epoxides
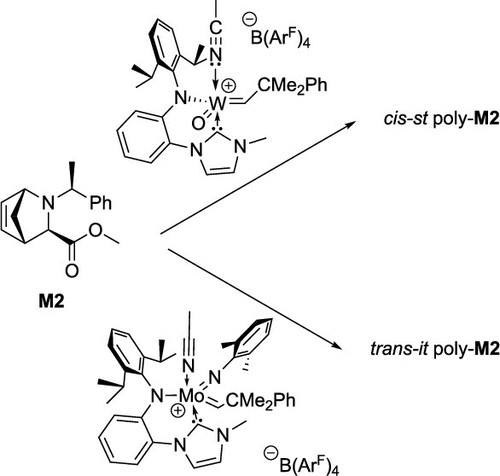
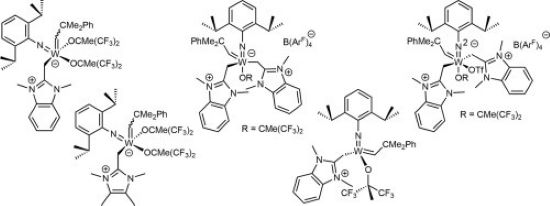
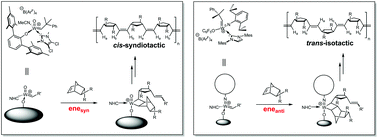
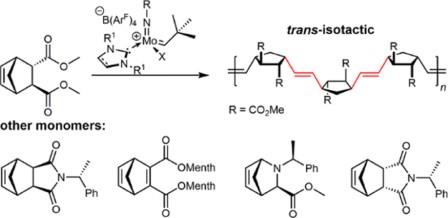
Olefin Metathesis and Stereoselective Ring-Opening Metathesis Polymerization with Neutral and Cationic Molybdenum(VI) Imido and Tungsten(VI) Oxo Alkylidene Complexes Containing N‑Chelating N‑Heterocyclic Carbenes
Manipulate – techniques to manipulate the surroundings of a synthetic catalyst to control activity and selectivity: general discussion
– GENERAL DISCUSSION – T. Beweries, M. R. Buchmeiser, N. R. Champness, M. Costas, A. Duhme-Klair, J. Echeverría, O. Eisenstein, C. T. J. Ferguson, J. C. Goodall, R. Gramage-Doria, M. Gyton, R. Ham, S. Herres-Pawlis, Ch. L. Johnson, P. Kennepohl, B. Lewandowski, P. R. Linnebank, S. A. Macgregor, K. T. Mahmudov, E. Meeus, M. Navarro, P. Ntola, T. N. Parac-Vogt, R. N. Perutz, A. Poater, D. C. Powers, S. Pullen, P. R. Raithby, Joost N. H. Reek, T. R. Ward, A. S. Weller and H. Wennemers
Faraday Discuss., 2023, 244, 96-118.
Make – underpinning concepts of the synthesis of systems where non-covalent interactions are important: general discussion
– GENERAL DISCUSSION – T. Beweries, M. R. Buchmeiser, F. E. Bugden, N. R. Champness, B. Chanbasha, M. Costas, J. Echeverria, O. Eisenstein, C. Ferguson, J. C. Goodall, R. Gramage-Doria, M. Greenhalgh, M. Gyton, R. Ham, P. Kennepohl, B. Lewandowski, W.-Ch. Liu, S. A. Macgregor, K. T. Mahmudov, E. Meeus, J. Morris, P. Ntola, T. N. Parac-Vogt, R. N. Perutz, A. Poater, D. Powers, P. R. Raithby, J. N. H. Reek, I. Riddell, T. R. Ward, A. S. Weller and H. Wennemers
Faraday Discuss., 2023, 244, 434-454.